Answer: The correct answer is Option A.
Step-by-step explanation:
Double displacement reaction is a type of reaction in which exchange of ions takes place. General equation for these reactions is:
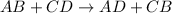
From the given options:
Option A: In the given reaction, sodium and hydrogen ions are getting exchanged. So, it is an example of double displacement reaction.
Option B: Here, copper and oxygen atoms are combining in their elemental state to form copper oxide. So, this is an example of synthesis reaction.
Option C: Here, iron and oxygen atoms are combining in their elemental state to form ferric oxide. So, this is an example of synthesis reaction.
Option D: In the given reaction, zinc is displacing hydrogen from its chemical reaction because it is more reactive metal. Hence, this reaction is an example of single displacement reaction.
Therefore, the correct answer is Option A.