Answer : There are 0.390 moles in 100 grams of S₈.
Explanation :
The formula to calculate mole is given below.
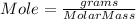
The molar mass of S₈ can be calculated as follows.
Molar mass of S₈ = 8 x atomic mass of S
Molar mass of S₈ = 8 x 32.06
Molar mass of S₈ is 256.48 g/mol
We have 100 grams of S₈.
Let us plug in these values in mole formula.
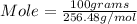
Mole = 0.390 mol.
0.390 moles are present in 100 grams of S₈