Answer:
Option C, 5 gram
Step-by-step explanation:
The half life of Cobalt-60
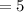 years
years
Given -
Sample of Cobalt-60
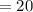 gram
gram
Time of radioactive decay
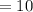 years
years
At the initial stage, when time T
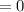 years, the mass of Cobalt-60
years, the mass of Cobalt-60
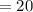 grams
grams
when time T
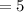 years, the mass of Cobalt-60
years, the mass of Cobalt-60
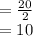 grams
grams
when time T
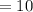 years, the mass of Cobalt-60
years, the mass of Cobalt-60
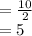 grams
grams
Hence, option C is correct.