Answer: non polar molecules
Step-by-step explanation:
An electronegative atom is the one which attracts the shared pair of electron towards itself.
A polar covalent molecule is defined as the molecule which is formed when there is a difference of electronegativities between the atoms. Example:
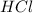
Non-polar covalent molecule is defined as the molecule which is formed when there is no difference of electronegativities between the atoms. It is also defined as the bond which is formed due to the equal sharing of electrons between the atoms. Example:
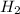
Hence, the correct answer is non polar covalent molecule.