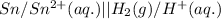Answer: The cell notation for the given cell will be:
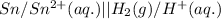
Step-by-step explanation:
For the given cell reaction:
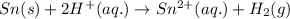
The half cell reaction follows:
Oxidation half reaction:
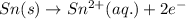
Reduction half reaction:
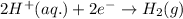
Oxidation reaction always occurs at anode.
Cell notation is the representation in which, the anode is written on left hand side followed by its ion with its molar concentration. It is then followed by a salt bridge, which is represented by the symbol "||" . Then the cathodic ion with its molar concentration is written and then the cathode.
Hence, the cell notation for the given cell will be: