Answer : The given equation represents "Substitution reaction" of alkanes
Explanation :
The given reaction is,
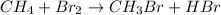
To find out the type of reaction, we need to look at the reactants and products involved in the reaction
CH₄ is an alkane known as methane and Br₂ is bromine which belongs to halogen family.
During the reaction , we can see that one of the H atoms of methane is substituted by Br to form a product CH₃Br.
Therefore this is a "substitution reaction of alkanes" Since a hydrogen atom gets replaced by a halogen atom, it is also termed as "halogenation of alkanes"