Answer:
91.728 cal required to increase the temperature 11 °C to 23 °C.
Step-by-step explanation:
Mass of the alcohol ,m= 13 g
The specific heat of the alcohol,c = 0.588 cal/g °C
Change in temperature =

Heat required to increase the temperature be Q.
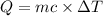
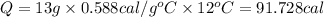
91.728 cal required to increase the temperature 11 °C to 23 °C.