Answer : 89 mL of solution would contain the given amount of Al(NO₃)₃.
Explanation :
Step 1 : Find moles of Al(NO₃)₃.
The molar mass of Al(NO₃)₃ is 213 g/mol
The formula to calculate mole is given below.
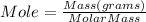
We have 12 g of Al(NO₃)₃. Let us plug in this value to find mol.
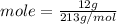
Mole = 0.056 mol.
We have 0.056 mols of Al(NO₃)₃
Step 2 : Use molarity formula to find the volume.
The molarity of a solution is defined as moles of solute per liter of solution.
This can be represented in terms of formula as follows.
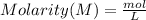
We have 0.63 M solution.
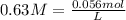
On rearranging we get,
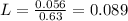
We have 0.089 L of solution. Let us convert this to mL.
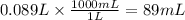
89 mL of solution would contain the given amount of Al(NO₃)₃.