Answer: Ions are conductive
Explanation: Acetic Acid is a strong electrolyte which in dissolution in aqueous solutions can produce its respective positive and negative species in the form of ions.
When Acetic Acid dissolves -
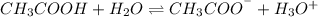
Thus the cations and anions are responsible for the conduction of electric current as in ionic solutions, ions can move freely when dissociated in the solution .