we can use the combined gas law equation as the amount of gas is constant in both instances.
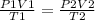
P - pressure , V- volume and T - temperature in kelvin
parameters at STP are on the left side and parameters for the second instance are on the right side of the equation
P1 - 101 325 Pa
T1 - 273 K
V1 - 15.0 L
P2 - 735 Torr x 133 Pa/Torr = 97 755 Pa
T2 - 57 °C + 273 = 330 K
substituting these values in the equation
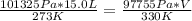
V = 18.8 L
the volume it occupies at the given conditions is 18.8 L