Answer:
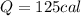
Step-by-step explanation:
Hello,
In this case, this could be determined via the following equation for the determination of the absorbed heat by the aluminium, considering the heat capacity, the change in the temperature and the heated mass:
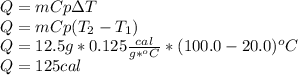
Best regards.