Answer : The correct option is B: The acid donates an H + ion.
Explanation :
According to Bronsted -Lowry theory, an acid is a substance that donates a proton. The substance that is left after the proton is donated is known as "conjugate base" of that acid.
Consider a general acid HA. The dissociation reaction of the acid can be written as follows.
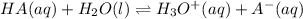
In the above example, HA behaves as an acid because it donates proton to H₂O. When the proton is donated, A⁻is formed on the product side which is a conjugate base of HA.
From above discussion, we can say that a substance and its conjugate differ by a proton, H⁺ only.
Therefore the acid donates an H + ion is the correct option.