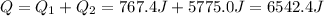There are two different processes here:
1) we must add heat in order to bring the temperature of the water from
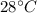
to
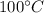
(the temperature at which the water evaporates)
2) other heat must be added to make the water evaporates
1) The heat needed for process 1) is
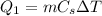
where
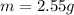
is the water mass
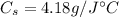
is the water specific heat
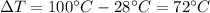
is the variation of temperature of the water
If we plug the numbers into the equation, we find
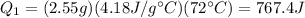
2) The heat needed for process 2) is
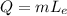
where
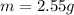
is the water mass
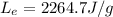
is the latent heat of evaporation of water
If we plug the numbers into the equation, we find
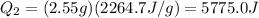
So, the total heat needed for the whole process is