Step-by-step explanation:
Mass of the strontium-90 present at initial stage=
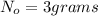
Mass of the strontium-90 present after time t = N = q
The half life of strontium-90 =
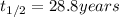
Decay constant =
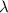
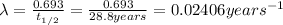
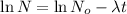
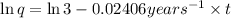
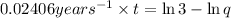
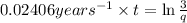
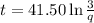
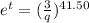
From the above expression we can see that t and q are inversely related to each other.