Answer : The molar mass of silver nitrate
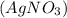 is, 169.87 g
is, 169.87 g
Explanation :
Molar mass : It is defined as mass of 1 mole of a substance.
Given:
Molar mass of Sliver (Ag) = 107.8682 g
Molar mass of nitrogen (N) = 14.0067 g
Molar mass of oxygen (O) = 15.9994 g
Now we have to calculate the molar mass of silver nitrate
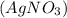
Molar mass of silver nitrate
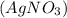 = Molar mass of Sliver (Ag) + Molar mass of nitrogen (N) + (3 × Molar mass of oxygen (O))
= Molar mass of Sliver (Ag) + Molar mass of nitrogen (N) + (3 × Molar mass of oxygen (O))
Molar mass of silver nitrate
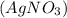 = 107.8682 g + 14.0067 g + 3(15.9994 g)
= 107.8682 g + 14.0067 g + 3(15.9994 g)
Molar mass of silver nitrate
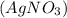 = 169.87 g
= 169.87 g
Therefore, the molar mass of silver nitrate
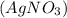 is, 169.87 g
is, 169.87 g