Answer:
There are 19.9 moles of phosphorus in
 atoms of phosphorus
atoms of phosphorus
Step-by-step explanation:
According to Avogadro´s number, 1 mol of each element has
 atoms of the same element, so:
atoms of the same element, so:
1 mol of phosphorus = 6.022
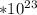 moles of phosphorus.
moles of phosphorus.
As we have
 moles of phosphorus, we need to find the relationship between this quantity of moles and the number of atoms using the Avogadro´s number:
moles of phosphorus, we need to find the relationship between this quantity of moles and the number of atoms using the Avogadro´s number:
 atoms of phosphorus *
atoms of phosphorus *
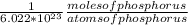 = 19.9 moles of phosphorus
= 19.9 moles of phosphorus