The correct answer is B) 2.
Note that the correctly balanced reaction should also have a factor 2 in front of RbCl(s). In fact, the balanced reaction is
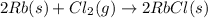
This way, we have:
- 2 atoms of Rb on the left side, and 2 atoms of Rb on the right side
- 2 atoms of Cl on the left side, and 2 atoms of Cl on the right side
so, the reaction is balanced.