Answer:
5.47 atm
Step-by-step explanation:
We have an initial state in which the air is at a temperature of 25 degrees and a pressure of 3.80 atm
We also have a final state in which the temperature is 36 degrees and we have to find out the pressure
For two different states it meets the following equation
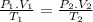
As we are told that the air is in a closed cylinder the volume is always constant, it is the same on both sides (
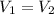 ) so we can simplify it and the equation is as follows
) so we can simplify it and the equation is as follows
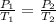
Now we clear the final pressure (
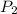 )
)
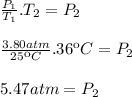
The final pressure is 5.47 atm