Answer:
mass of iron = 3319 g
Step-by-step explanation:
To find the mass of iron, we can use the following formula:
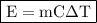 ,
,
where:
• E = energy transferred,
• m = mass,
• C = specific heat capacity, and
• ΔT = change in temperature,
and we can use it to solve for m.
But before that, we must convert the energy transferred from calories to Joules, since the specific heat capacity is given in units J/g°C. To convert from calories to Joules, we multiply by 4.184.
∴ 9550 cal = 9550 × 4.184
= 39957.2 J
We are told in the question that 9550 cal are removed, therefore E = -39957.2 J. We are also told that the temperature dropped from 100.0°C to 73.25 °C, therefore ΔT = 73.25 - 100.0 = -26.75°C.
Using this information, and the equation above, we can calculate the mass of the iron:
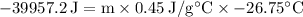
⇒
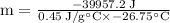
⇒ m = 3319 g
Therefore, the mass of the iron is 3319 g.