False.
In fact, the temperature T of a system is directly proportional to the internal energy U of the system, and the first law of thermodynamics states that the variation of internal energy of a system is given by
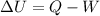
where
Q is the heat added to the system
W is the work done by the system on the surroundings
We see from the formula that we have 2 possible cases:
1) the heat added to the system (Q) is greater than the work done by the system (W) -->
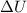
is positive, this means that the temperature of the system increases
2) the heat added to the system (Q) is less than the work done by the system (W) -->
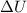
is negative, this means that the temperature of the system decreases
Therefore, it's not true that when heat is added to the system, its temperature must increase: it depends on the value of the work done, W. So, the original statement is false.