Answer : The specific heat of the substance is 0.0936 J/g °C
Explanation :
The amount of heat Q can be calculated using following formula.
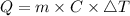
Where Q is the amount of heat required = 300 J
m is the mass of the substance = 267 g
ΔT is the change in temperature = 12°C
C is the specific heat of the substance.
We want to solve for C, so the equation for Q is modified as follows.
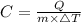
Let us plug in the values in above equation.
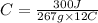
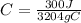
C = 0.0936 J/g °C
The specific heat of the substance is 0.0936 J/g°C