Attached is the MOT of NO molecule.
1. Bond order is calculated is
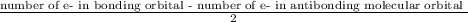
In present case, number of e- in bonding orbital = 6
number of e- in anti-bonding orbital = 1
∴ Bond-order =
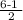
= 2.5
2. From the attached figure, it can be seen that NO has one unpaired electron in π* orbital.