Step-by-step explanation:
Molarity is defined as number of moles of solute present in liter of solution.
Mathematically, Molarity =

It is known that 1000 mL equals 1 L so, 350 ml equals 0.35 L. Therefore, calculate no. of moles as follows.
Molarity =

0.020 M =

no. of moles = 0.007 mol
Also, it is known that no. of moles equal mass divided by molar mass. Molar mass of NaCl is 58.44 g/mol.
Thus, calculate mass of NaCl as follows.
No. of moles =
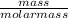
0.007 mol =

mass = 0.409 g
Thus, we can conclude that 0.409 grams of NaCl are present in 350 ml of 0.020 m nacl solution.