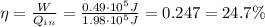b) The heat absorbed by the engine is
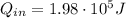
while the heat expelled is
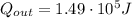
, therefore the work done by the engine is the difference between the heat absorbed and the heat expelled:
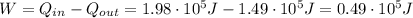
a) The efficiency of the engine is the ratio between the work done by the engine and the heat absorbed, therefore: