Answer : There are 6.71 moles of H₃PO₄.
Explanation:
The mole of a substance is calculated as

Let us find the molar mass of H₃PO₄.
Molar mass of H₃PO₄ = 3 x atomic mass of H + atomic mass of P + 4x atomic mass of O
From the periodic table, we have
Atomic mass of H = 1.008 g/mol
Atomic mass of P = 30.973 g/mol
Atomic mass of O = 15.999 g/mol
Molar mass of H₃PO₄ =
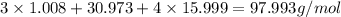
Molar mass of H₃PO₄ is 97.993 g/mol
The mass of H₃PO₄ is given as 658 g.
The moles can be calculated as,
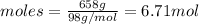
There are 6.71 mol in the given amount of H₃PO₄