Answer : 10.45 kJ of heat was transferred.
Explanation :
The equation we need to use here is given as,
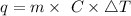
Where q is the amount of heat transferred
m is the mass of the water
C is the specific heat of water
ΔT is the change in temperature
Let us find out what information is given to us.
m = 100 g
The temperature change can be calculated as,
ΔT = final temperature - initial temperature
ΔT = 50°C - 25°C = 25°C
C = 4.18 J/g-°C
Let us plug in the above values to find q.

q = 10450 J
Let us convert this to kJ
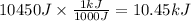
The amount of heat transferred was 10.45 kJ