Given that,
A system dissipates 12 J of heat into the surroundings.
28 J of work is done on the system.
To find,
The internal energy of the system.
Solution,
The first law of thermodynamics is used here. According to this law,
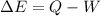
Q is heat and W is work done
Here,
Q = -12 J is the heat dissipated by the system
W = -28 J is the work done on the system
ATQ,
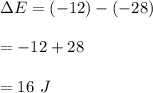
So, the change of internal energy of the system is 16 J.