a. Gas A has the lowest rate of diffusion
Further explanation
Given
The molar masses
Required
the lowest rate of diffusion
Solution
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
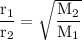
So :
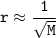
The greater the molar mass, the smaller the rate
So gas A has the lowest rate