Ionization of an acid of form (HA) can be represented as follows
HA ↔ H+ + A-
Ka =
![([H+][A-])/([HA])](https://img.qammunity.org/2019/formulas/chemistry/college/x7fvw6vbodjkw678pqy2i8jdheuk8fjzgr.png)
Now, initial conc. of HA = 0.12 M
Of these, 2.3 % got ionized.
∴ [H+] = [A-] = 0.12 X 0.023 = 0.00276 M
Thus [HA] left after ionization = 0.12 - 0.00276 = 0.11724 M
Now Ka =
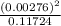
= 6.497 x 10^-5