The given question is incomplete. The complete question is:
Suppose a current of 0.920 A is passed through an electroplating cell with an aqueous solution of agno3 in the cathode compartment for 47.0 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell.
Answer: 0.0484 g
Step-by-step explanation:
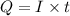
where Q= quantity of electricity in coloumbs
I = current in amperes = 0.920 A
t= time in seconds = 47.0 sec
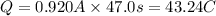
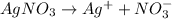
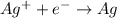
96500 Coloumb of electricity electrolyzes 1 mole of Ag
43.24 C of electricity deposits =
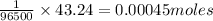 of Ag
of Ag
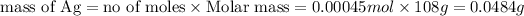
Thus the mass of pure silver deposited on a metal object made into the cathode of the cell is 0.0484 g