Molarity is the method of expressing concentration of solution. Mathematically, it is expressed as
Molarity =
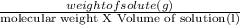
In present case, Molecular weight of CaCl2 = 110.98 g
volume of solution = 750 ml = 0.750 l
weight of solute (CaCl2) = 45 g
∴Molarity =
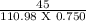
= 0.54 M
Concentration of solution is 0.54 M