Molarity is one of the method of expressing concentration of solution. Mathematically it is expressed as,
Molarity =
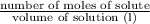
Given: Molarity of solution = 5.00 M
Volume of solution = 750 ml = 0.750 l
∴ 5 =
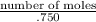
∴
number of moles = 3.75Answer: Number of moles of KOH present in solution is 3.75.