if theres a mixture of components we can calculate the mole fraction
mole fraction can be calculated as follows
mole fraction of component =
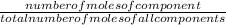
number of moles of ethanol - 3.00 mol
total number of moles in mixture - 3.00 + 5.00 = 8.00 mol
mole fraction of ethanol =
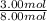
mole fraction of ethanol is 0.375