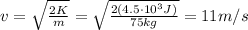A) The average translational kinetic energy of the molecules in a gas is given by:
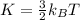
where
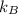
is the Boltzmann's constant
T is the absolute temperature of the gas
In our problem,
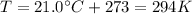
, so the average translational kinetic energy of the molecules is
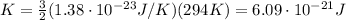
We have 1.2 mol of this gas, and since one mole of ideal gas contains a number of molecules equal to Avogadro number, the total number of molecules in our gas is
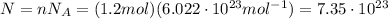
So the total translational kinetic energy of all molecules of the gas is
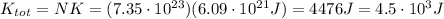
B) The kinetic energy of a person is given by:
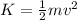
where m is the person's mass and v his velocity. The person has a mass of m=75 kg and its energy is equal to the energy of the gas,
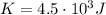
, therefore his velocity must be