Following reaction takes place between LiOH and HNO3
LiOH + HNO3 → LiNO3 + H2O
Thus, 1 mole of LiOH reacts with 1 mole of HNO3 to form 1 mole of LiNO3.
Now,
Number of millimoles of LiOH consumed =
36.90 X 0.100 = 3.690
∴Number of millimoles of HNO3 present = 3.690
Now, Molarity of HNO3 =
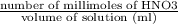
=
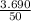 = 0.0738 M
= 0.0738 M
Thus, molarity of the HNO3 solution is 0.0738 M