Answer: The pressure of the container will be 0.796 atm.
Step-by-step explanation:
To calculate the mass of bromine gas, we use the ideal gas equation, which is:
PV = nRT
where,
P = pressure of the gas = ? atm
V = Volume of the gas = 7.5 L
n = Number of moles of gas = [0.125 + 0.125] = 0.25 mol
R = Gas constant =
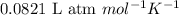
T = temperature of the gas =
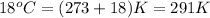
Putting values in above equation, we get:
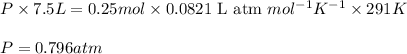
Hence, the pressure of the container will be 0.796 atm.