Answer : The correct answer is 1.64 * 10³ Kg.
Given : Mass of CaCl₂ = 5.00 * 10³ Kg
Converting Kg to g using ( 1 Kg = 1000 g)
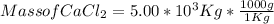
Mass of CaCl₂ = 5.00 * 10⁶ g
Following steps can be done :
Step 1) To write a balanced reaction :
The reaction between Calcium Carbonate and Hydrochloric acid as:
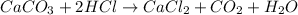
Step 2 ) To find mole of CaCl₂ .
Mass of compound can be used to calculate mole by Mole formula as :
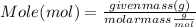
Mass of CaCl₂ = 5 * 10⁶ g Molar mass of CaCl₂ = 110.98
Plugging values in formula
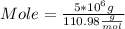
Mole of CaCl₂ = 4.5 * 10^4 mol
Step 3) To write mole ratio of HCl : CaCl₂
Mole ratio is found from balanced reaction .
Mole of HCl in balanced reaction = 2 mol
Mole of CaCl₂ in balanced reaction = 1 mol
hence mole ratio of HCl : CaCl₂ = 2 : 1
Step 4) To find mole of HCl
Mole of HCl can be calculated using mole of CaCl₂ and mole ratio as :
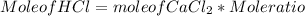
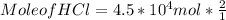
Mole of HCl = 9.0 * 10⁴ mol
Step 5) To find mass of HCl
Mass of HCl can be calculates using its mole by Mole formula as:
Molar mass of HCl = 36.5
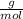
Mole of HCl = 9.0 * 10⁴ mol
Plugging values in Mole formula as:
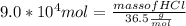
Multiplying both side by
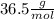
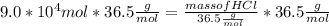
Mass of HCl = 3.285 * 10⁶ g
Step 6) Convert mass of HCl from g to Kg
1 Kg = 1000 g
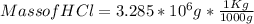
Mass of HCl = 3.285 * 10³ Kg
Hence mass of HCl required to produce 5.00 * 10³ Kg of CaCl₂ is 3.285 *10³Kg.