Answer:
The correct answer is: C–H bonds in methane and O–O bonds in oxygen.
Step-by-step explanation:
During the combustion of methane, methane react with oxygen molecule to give carbon dioxide and water molecule.
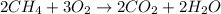
During the course of reaction :
- Bond between C-H atom in methane will be broken down
- Bond between O-O bond will be broken down.
During course of formation of products:
- Bonds between oxygen and carbon atom will formed
- Bonds between oxygen ah hydrogen at will be formed.