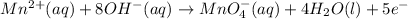Answer : The balanced oxidation half reaction in basic medium will be :
Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Rules for the balanced chemical equation in basic solution are :
- First we have to write into the two half-reactions.
- Now balance the main atoms in the reaction.
- Now balance the hydrogen and oxygen atoms on both the sides of the reaction.
- If the oxygen atoms are not balanced on both the sides then adding water molecules at that side where the more number of oxygen are present.
- If the hydrogen atoms are not balanced on both the sides then adding hydroxide ion
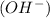 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present. - Now balance the charge.
The balanced oxidation half reaction in basic medium will be :