Number of millimoles of NaOH consumed in present titration =
33.23 X 0.09892
= 3.287
Thus, number of millimoles of vinegar present in sample = 3.287
Now, Molarity of vinegar solution =
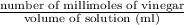
=
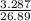
= 0.1222 mol/dm3
Molar concentration of vinegar present in sample solution is 0.1222 M