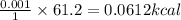Answer: 0.0612 kcalories
Explanation:
Endothermic processes are those processes in which heat is absorbed and exothermic processes are those processes in which heat is released.
Standard units for heat measurement are Joules and calories wherein
1 joule = 0.239 calorie.
As 1 Joule is equivalent to 0.239 calorie
256 Joules are equivalent to=
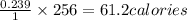
Now 1 cal = 0.001 kcalories
Therefore 61.2 calories=