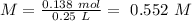Answer:
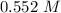
Step-by-step explanation:
For the calculation of molarity "M" we have start with the molarity equation:
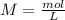
So, we have to calculate the moles of
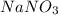 and the L of
and the L of
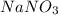 .
.
For the calculations of moles we have to use the molar mass of
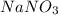 .
.
Na=23 g/mol
N=14 g/mol
O= 16 g/mol
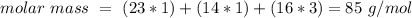
or
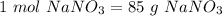
Now, we can find the moles of
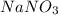 :
:
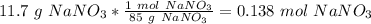
The next step would be the converstion from mL to L:
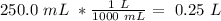
Finally, we have to plug both values in the molarity equation: