Answer:
0.668 g of barium sulfate
Step-by-step explanation:
Given,
Balanced chemical equation: BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl.
Volume of BaCl₂ = 25.34 mL x
 = 0.02534 L.
= 0.02534 L.
Molarity of BaCl₂ = 0.113 M
Molarilty =
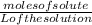
Moles of solute = Molarilty x L of the solution
Moles of BaCl₂ = 0.113 M x 0.02534 L = 0.00286 mol.
From the balanced chemical equation there is a 1:1 molar ratio between BaCl₂ and BaSO₄
Therefore, moles of BaCl₂ = moles of BaSO₄
Moles of BaSO₄ = 0.00286 mol.
Mass of BaSO₄ = moles of BaSO₄ x Molar mass of BaSO₄
Mass of BaSO₄ = 0.00286 mol x 233.4 g/mol.
Mass of BaSO₄ = 0.668 g.