Answer: Four times
Step-by-step explanation:
Given
If Pressure and Volume is double
For ideal gas
PV=nRT
where P=Pressure
V=volume
n=no of moles
R=gas constant
T=temperature
Initially
PV=nRT......(i)
after doubling
(2P)(2V)=nRT'.......(ii)
divide (i) and (ii)
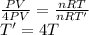
So, Temperature becomes 4 times