Answer:
238.91 g/mol is the molar mass of the solute.
Step-by-step explanation:
Mass of solute = 5.65 g
Molar mas of solute = M
Mass of solvent = 110.0 g = 0.110 kg
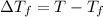
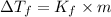
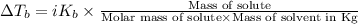
where,
 =Depression in freezing point
=Depression in freezing point
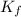 = freezing point constant of solvent= 5.07°C/m
= freezing point constant of solvent= 5.07°C/m
1 =van't Hoff factor
m = molality
Freezing point constant of benzene ,T= 5.45°C/m
T = 5.45°C ,
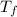 =4.39 °C
=4.39 °C
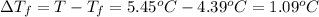
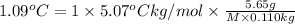
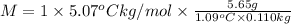
i = 1
M = 238.91 g/mol
238.91 g/mol is the molar mass of the solute.