Osmotic pressure is mathematically expressed as
π = CRT
where, C = concentration of solution
R = gas constant = 0.082 atm mol-1 K-1
T = temperature = 300 K
π = osmotic pressure = 0.55 atm
∴ 0.55 = C X 8.314 X 300
∴C = 0.022 M
Now, conc of solution (in terms of molarity) =
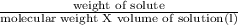
Given: weight of solute = 0.9 g
volume of solution = 100 ml = 0.1 l
∴ 0.022 =
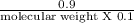
∴ Molecular weight = 4.09 g/mol