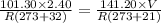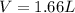Answer:
V = 1.66 L
Step-by-step explanation:
As we know that at the depth of 10 m the volume of the lungs of diver is given as
V = 2.40 L
Temperature = 32 degree C
Pressure = 101.30 kPa
Now we have change the values of pressure and temperature
Pressure = 141.20 kPa
Temperature = 21 degree C
Now we can use idea gas equation to solve this
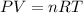
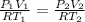
so we have