We will use this law
PV = nRT
we have to convert pressure from kPa to atm
1 atm = 101.325 kPa
? atm = 112.1 kPa
P = 1.11 atm
T = 30.8 °C + 273.15 = 303.95 K
V = 8.86 L
R = 0.088205 atm. L / mol . Kelvin
n =
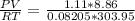
= 0.39 mol
number of N₂ molecules = number of moles * Avogadro's number
= 0.39 * (6.022 * 10²³) = 2.37 x 10²³ molecules