Answer: The two correct statements are the concentration of one or more of the reactants is small and the reaction will proceed to the right and favor the formation of products.
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power its stoichiometric coefficients. It is expressed as
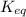
For a general chemical reaction:
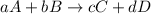
The expression for
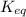 is given as:
is given as:
![K_(eq)=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2019/formulas/chemistry/college/8i6z9kdl5sbqqw6gt2ligbic9v48km7t6n.png)
Equilibrium constant is inversely related to the concentration of the reactants. So, if concentration of any reactants is less, the vale of
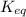 will be more.
will be more.
Conditions of
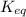 are:
are:
- When K < 1; the reaction is favored to the left or in backward direction or reactants are formed more.
- When K > 1; the reaction is favored to the right or in forward direction or products are formed more.
- When K = 1; the reaction is in equilibrium.
Hence, the two correct statements are the concentration of one or more of the reactants is small and the reaction will proceed to the right and favor the formation of products.