Answer: 0.407 moles of aluminum chloride could be produced from 11.0 g of aluminum.
Step-by-step explanation:
To calculate the moles :
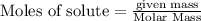
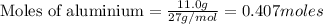
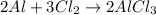
According to stoichiometry :
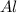 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
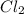 is the excess reagent.
is the excess reagent.
As 2 moles of
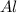 give = 2 moles of
give = 2 moles of
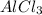
Thus moles of
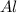 give =
give =
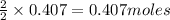 of
of
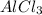
Thus 0.407 moles of aluminum chloride could be produced from 11.0 g of aluminum.